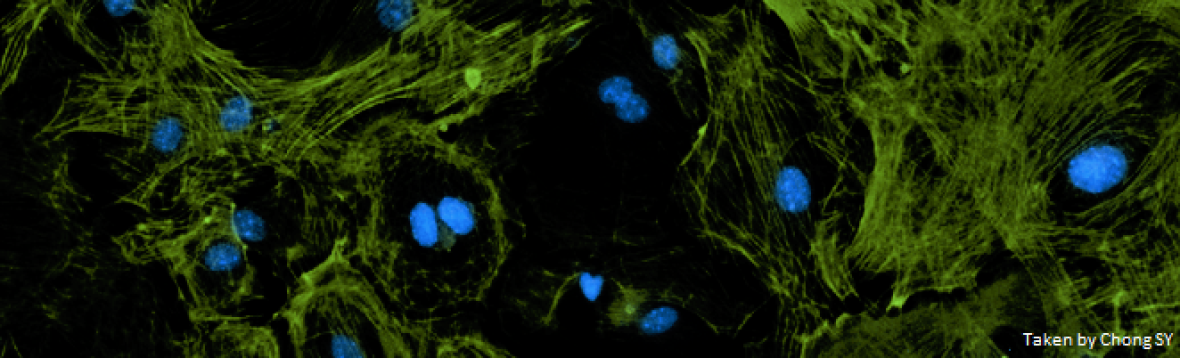
Our team has been working on cardio-metabolic diseases, cardiac immunology, extracellular vesicles and nanomedicine. Our laboratory is working with various established pre-clinical animal models of human disease, including myocardial infarction, ischemia reperfusion injury, pressure overload, diet-induced heart failure), atherosclerosis and non-alcoholic fatty liver disease. The newly established NANONASH research program aims to tap unto brilliant and young scientists to develop new and effective nanomedicine-based therapies for the treatment of fatty liver disease. Our group also works on new nanomaterials and drug delivery systems (carrying small molecules, nucleic acids, RNAs etc) for translational research in cardiovascular disease, fatty liver disease and gut related disease. Our nanomedicine laboratory, the Centre for NanoMedicine, provides state-of-the-art technology to enable such research and expand the applications of nanomedicine for unmet clinical needs.
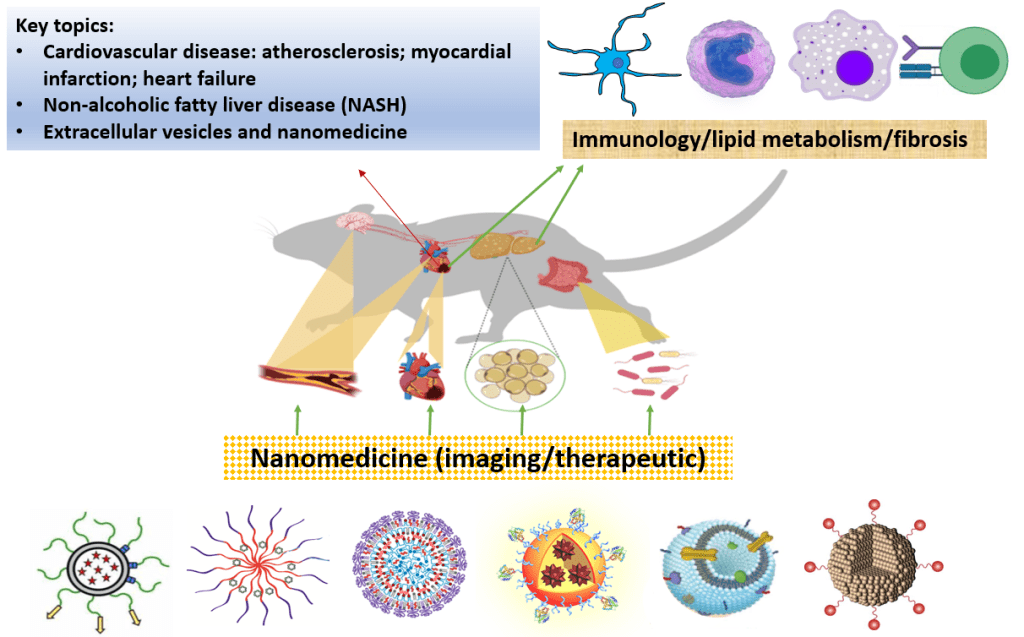

Our team aims to :
- Unravel novel biomarker panels for early disease detection.
- Discover disease mechanisms and identity novel therapeutic targets in cardio-metabolic diseases.
- Employ clinically-relevant disease models and nanoparticles of various classes to develop safe, effective and clinically translatable (theranostic) nanomedicines.
Our capabilities :
- Animal models of diseases
- State-of-the-art in vitro and in vivo imaging
- Advanced flow cytometry and FACS
- Extracellular vesicles
- Nanoparticle formulation and characterization
Research Highlights :
(click on the title for detailed information):
New study shows effectiveness of nanoparticles in diagnosing, treating plaque in arteries
Featured in The Straits Times
These innovative nanoparticles are designed to break down in the acidic environment of atherosclerotic plaques, releasing gadolinium, a contrast agent that enhances visualization in magnetic resonance imaging (MRI). This enables real-time imaging to assess plaque severity more effectively.
Tiny but powerful : Can milk particles be therapy for gut disease?
Milk nanoparticle is believed to be the therapy for gut disease. They are a thousand times smaller than the width of a human hair and can be seen only with an electron microscope. Treatment with antibiotics, anti-inflammatory drugs or immunosuppressive drugs comes with side effects and antibiotics, for instance, can disturb gut balance and damage beneficial bacteria. These tiny milk particles can pass through the gut and survive the stomach’s gastric environment to reach the intestines. They go to the disease sites “more specifically” and avoid healthy tissue which reduces the side effects and increases the treatment efficacy.

Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies
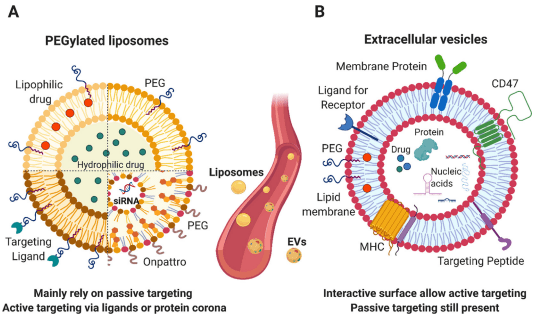
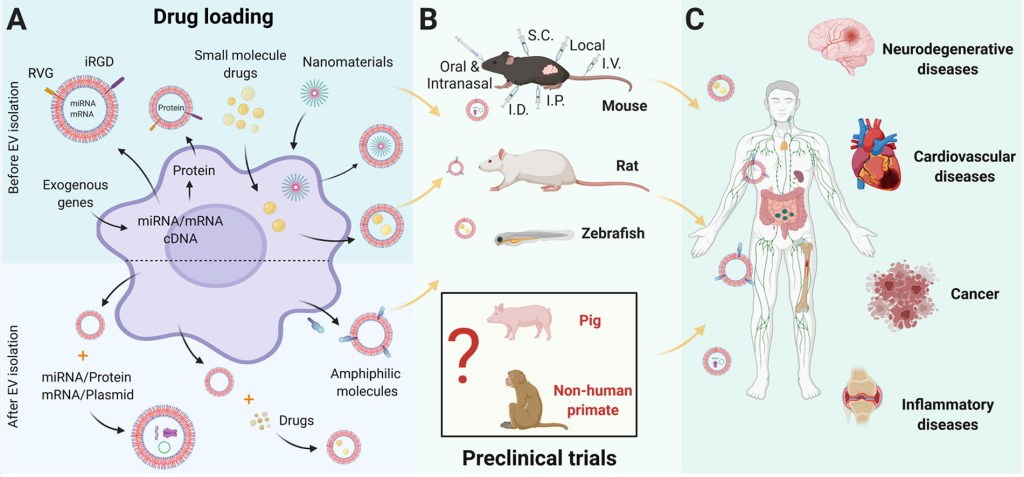
During the past decades, extracellular vesicles (EVs) have emerged as an attractive drug delivery system. In this review, the pre-clinical applications of EVs were accessed. Based on the trend observed from study published over the past decade, we have define the niches where to apply EVs for drug delivery in the future and to provide a basis for regulatory guidelines.
(Featured inNUSMed Press Release; Reported by 新加坡联合早报(zaobao),BioMed 科技)
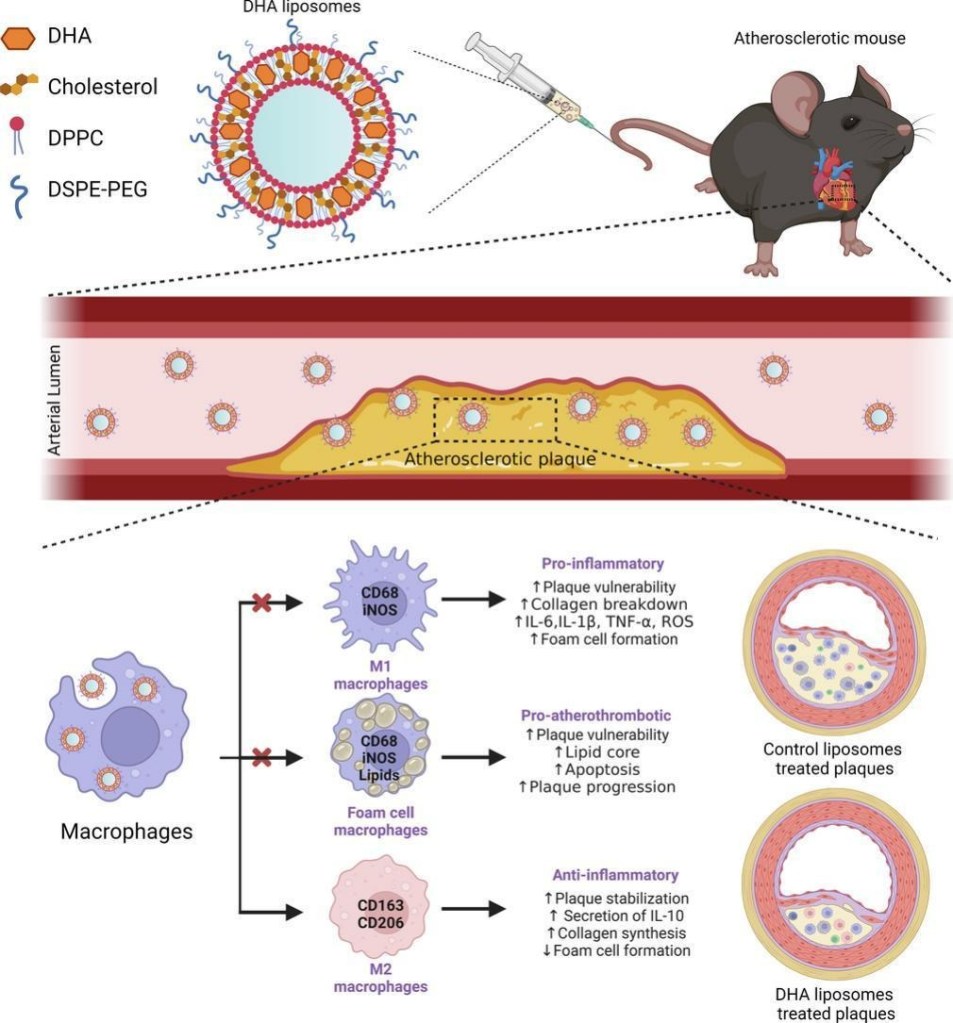
Docosahexaenoic acid (DHA, 22:6n-3), an omega-3 polyunsaturated fatty acid (PUFA), which exerts anti-inflammatory and antioxidant properties, has long been purported to be of therapeutic benefit to atherosclerosis patients. However, large clinical trials have yielded inconsistent data, likely due to variations in the formulation, dosage, and bioavailability of DHA following oral intake. To fully exploit its potential therapeutic effects, we have developed an injectable liposomal DHA formulation intended for intravenous administration as a plaque-targeted nanomedicine. The liposomal formulation protects DHA against chemical degradation and increases its local concentration within atherosclerotic lesions.
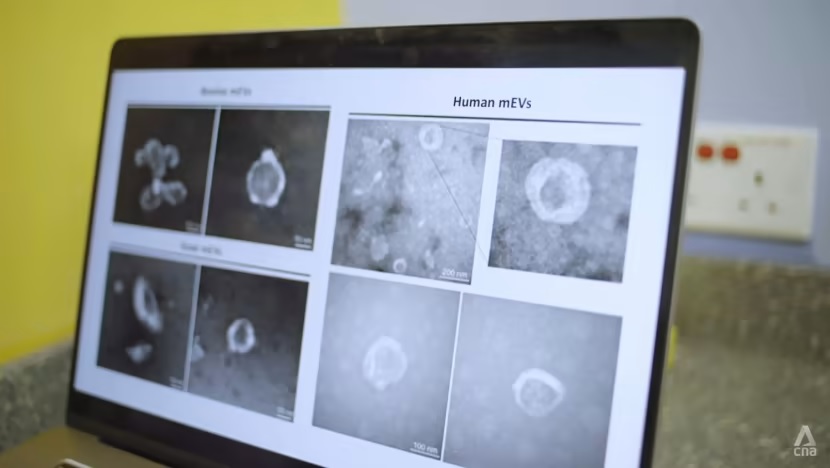
Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis
(Reported by CNA (Channel NewsAsia), NEWS AZI, EurekAlert, NUS Press Release, X-MOL, PHYS.ORG, MIRAGE NEWS)
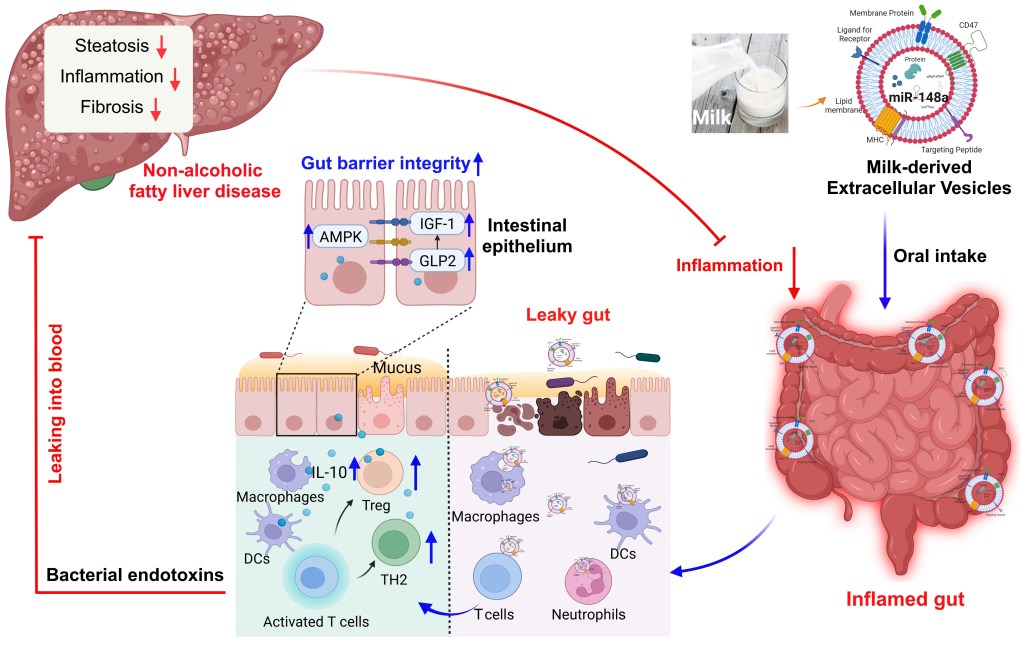
Milk-derived extracellular vesicles (mEVs) have been proposed as a potential nanomedicine for intestinal disorders; however, their impact on intestinal barrier integrity in gut inflammation and associated metabolic diseases has not been explored yet. Here, mEVs derived from bovine and human breast milk exert similar protective effects on epithelial tight junction functionality in vitro, survive harsh gastrointestinal conditions ex vivo, and reach the colon in vivo. Oral administration of mEVs restores gut barrier integrity at multiple levels, including mucus, epithelial, and immune barriers, and prevents endotoxin translocation into the liver in chemical-induced experimental colitis and diet-induced nonalcoholic steatohepatitis (NASH), thereby alleviating gut disorders, their associated liver inflammation, and NASH. Oral administration of mEVs has potential in the treatment of gut inflammation and gut-liver axis–associated metabolic diseases via protection of intestinal barrier integrity.
Featured by Channel NewsAsia and Interviewed by CNA Insider.
Bovine milk constitutes an essential part of human diet, especially for children, due to its enrichment of various nutrients. We recently developed an effective protocol for the isolation of extracellular vesicles from milk (mEVs) and discovered that mEVs contained large amounts of immune-active proteins and modulated the gut immunity and microbiota in healthy mice. Here, we aimed to explore the therapeutic effects of mEVs on inflammatory bowel disease.
Press Release; Highlighted by ACS材料X
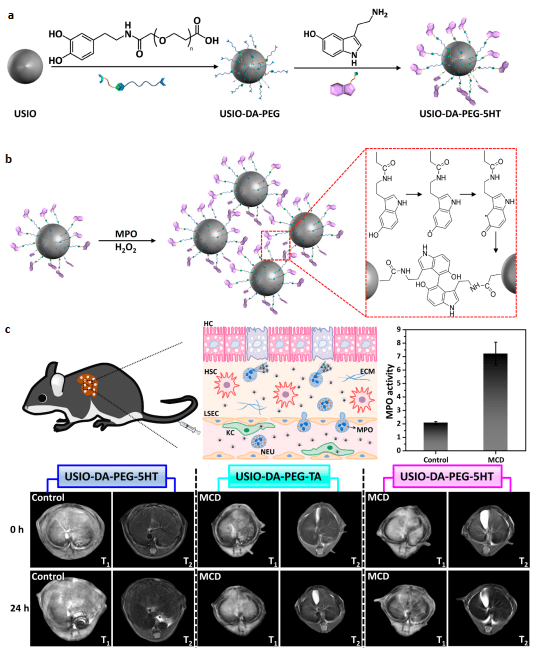
The diagnosis of nonalcoholic steatohepatitis (NASH) is important for preventing the progress of nonalcoholic fatty liver disease (NAFLD) into a fatal condition. Considering the expression of oxidative enzyme myeloperoxidase (MPO) which is a key player in lipid peroxidation in inflamed tissues, was much higher in NASH than in the nonalcoholic fatty liver (NAFL) with steatosis, we designed a nanoparticle platform based on ultrasmall iron oxide (USIO) nanoparticles to realize MPO-sensitive NASH diagnosis. These USIO-DA-PEG-5HT nanoprobes offer great potential for improving NASH MR imaging diagnostic accuracy and sensitivity compared to existing molecular MR contrast agents with a single imaging modality.
Reported by X-MOL

The coagulation protein tissue factor (TF) regulates inflammation and angiogenesis via its cytoplasmic domain in infection, cancer and diabetes. While TF is highly abundant in the heart and is implicated in cardiac pathology, the contribution of its cytoplasmic domain to post-infarct myocardial injury and adverse left ventricular (LV) remodeling remains unknown.
Featured by Editorial Highlight
The Toll-like receptor 7 (TLR7) is an intracellular innate immune receptor activated by nucleic acids shed from dying cells leading to activation of the innate immune system. Since innate immune system activation is involved in the response to myocardial infarction (MI), this study aims to identify if TLR7 is involved in post-MI ischaemic injury and adverse remodelling after MI. Our findings revealed that in acute MI, TLR7 mediates the response to acute cardiac injury and chronic remodelling probably via modulation of post-MI scar formation and BM-derived inflammatory infiltration of the myocardium.
Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction
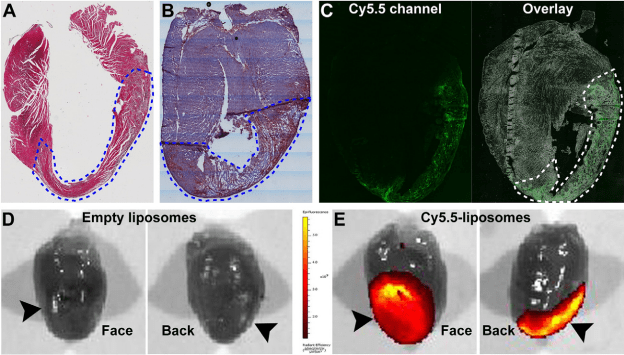
Inflammation is a known mediator of adverse ventricular remodeling after myocardial infarction (MI) that may lead to reduction of ejection fraction and subsequent heart failure. Berberine has been associated with anti-inflammatory, anti-oxidative, and cardioprotective properties. However, due to its poor solubility in aqueous buffers and its short half-life in the circulation upon injection, the extensive usage of this natural product have been hampered. We hypothesized that encapsulation of berberine into long circulating liposomes could improve its therapeutic availability and efficacy by protecting cardiac function against MI in vivo.